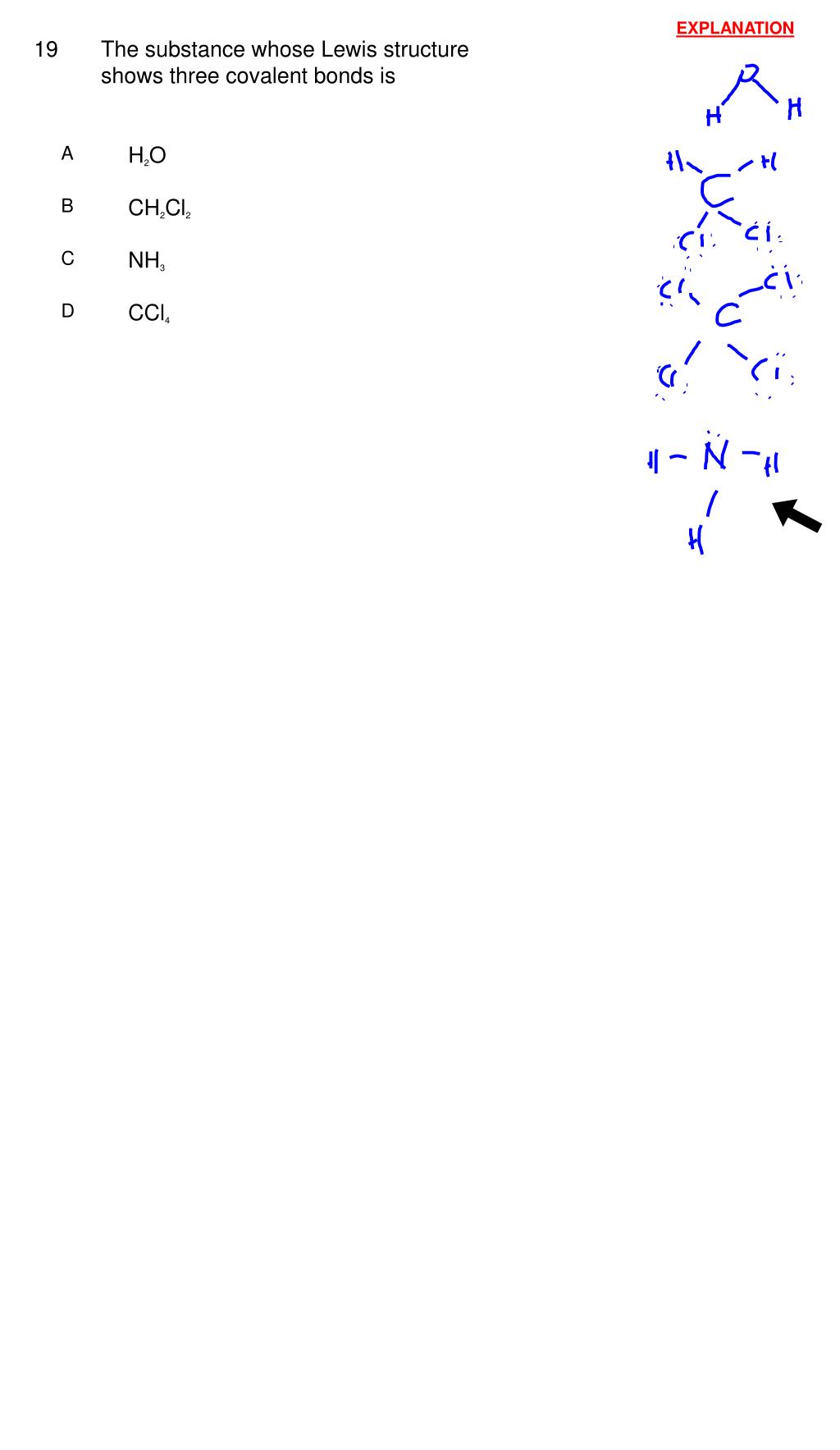
The Substance Whose Lewis Structure Shows Three Covalent Bonds Is
This can easily be observed in a. Intermolecular forces are the weak forces of attraction found between the individual molecules of a molecular covalent substance.

Test 2 Pretest Objective Administer This Practice Test
Water is a tasteless odorless liquid at ambient temperature and pressureLiquid water has weak absorption bands at wavelengths of around 750 nm which cause it to appear to have a blue colour.

. Chemical Bond Questions and Answers. For many chemical species a single Lewis structure consisting of atoms obeying the octet rule possibly bearing formal charges and connected by bonds of positive integer order is sufficient for describing. Covalent bonds occur between identical atoms or between different atoms whose.
For molecules a Lewis structure in which all of the formal charges are _____ is preferred to one that contains _____ formal charges. Although the quantity of novel and advanced porous functional materials has been exploded in recent years constructing persistent porous structures connected by covalent bonds is still a formidable task The multifunctionality of the framework chemistry can achieve. Covalent bonds are much more common in organic chemistry than ionic bonds.
Although the information necessary for life to go on is encoded by the DNA molecule the dynamic process of life maintenance replication defense and reproduction are carried out. Solid-liquid liquid-gas and solid-gas. A molecule whose Lewis structure could be described by more than one resonance structure possesses an electronic structure equal to that of a combination of the resonance structures with the more stable structures contributing more to the average structure.
The VSEPR theory predicts that the geometry around each oxygen atom in H 2 O 2 should be bent. A covalent bond consists of the simultaneous attraction of two nuclei for one or more pairs of electrons. These involve atoms in the polypeptide backbone as well as atoms in the amino acid side chains.
Under the framework of valence bond theory resonance is an extension of the idea that the bonding in a chemical species can be described by a Lewis structure. Access the answers to hundreds of Chemical bond questions that are. Proteins are molecular devices in the nanometer scale where biological function is exerted 1.
For example a carbon atom weighs less than 2 10 23 g and an electron has a charge of less than 2 10 19 C coulomb. The Lewis structure of hydrogen peroxide contains an O-O single bond as shown in the figure below. A Lewis structure shows the number of covalent bonds between bonded atoms.
Compounds containing covalent bonds are part and parcel of our day-to-day life. The electrons located between the two nuclei are bonding electrons. Organic Chemistry Questions and Answers.
From cooking gas to the sugar in lemonade from the oxygen we inhale to the exhalation of carbon dioxide all consist of compounds containing covalent bonds. The folding of a protein chain is however further constrained by many different sets of weak noncovalent bonds that form between one part of the chain and another. The temperature and pressure conditions at which a substance exists in solid liquid and gaseous states are summarized in a phase diagram for that substance.
Get help with your Chemical bond homework. Get help with your Organic chemistry homework. Consider the phase diagram for carbon dioxide shown in Figure 5 as another example.
From the water used to boil an egg to the protein present inside it all are compounds having a covalent bond. But this theory cannot predict whether the four atoms should lie in the same plane or whether the molecule should be visualized as lying in two. When describing the properties of tiny objects such as atoms we use appropriately small units of measure such as the atomic mass unit amu and the.
They are the building blocks of all cells in our bodies and in all living creatures of all kingdoms. Water is the chemical substance with chemical formula H 2 O. We would like to show you a description here but the site wont allow us.
Notice that the triple point is well above 1 atm indicating that carbon dioxide. Hydrogen bonds ionic bonds and van der Waals attractions as explained in. Access the answers to hundreds of Organic chemistry questions that are.
Phase diagrams are combined plots of three pressure-temperature equilibrium curves. The solid-liquid curve exhibits a positive slope indicating that the melting point for CO 2 increases with pressure as it does for most substances water being a notable exception as described previously. One molecule of water has two hydrogen atoms covalently bonded to a single oxygen atom.
The weak bonds are of three types. Atomsand the protons neutrons and electrons that compose themare extremely small. Porous materials are increasingly important in technology and applications.

Test 2 Pre Test Grade Grade Subject Test 2 Ch 2 9 Date Date Objective Administer This Practice Test 2 Thoroughly Explain All Incorrect Answers Ppt Download

Test 2 Pre Test Grade Grade Subject Test 2 Ch 2 9 Date Date Objective Administer This Practice Test 2 Thoroughly Explain All Incorrect Answers Ppt Download
Ppt Test 2 Pre Test Powerpoint Presentation Free Download Id 4526227
No comments for "The Substance Whose Lewis Structure Shows Three Covalent Bonds Is"
Post a Comment